 Short Answer Type
Short Answer TypeWhat happens when:
(i) Ethyl alcohol is heated in the presence of Al2O3 at 493 K?
(ii) Ethylene dibromide is heated with zinc dust?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhy do alkenes undergo electrophilic addition reactions?
Alkenes undergo electrophilic addition reaction. The 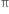 electrons constituting the
electrons constituting the 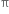 bond in the double bond are more mobile, easily detachable and more easily available for the chemical reaction. This means >C = C< can act as a source of electrons. There are certain reagents (electrophiles) which are capable of adding to alkene molecules forming an addition compound. Since such reactions are initiated by the electrophiles, hence are known as Electrophilic addition reactions.
bond in the double bond are more mobile, easily detachable and more easily available for the chemical reaction. This means >C = C< can act as a source of electrons. There are certain reagents (electrophiles) which are capable of adding to alkene molecules forming an addition compound. Since such reactions are initiated by the electrophiles, hence are known as Electrophilic addition reactions.
The mechanism proceeds in two steps:
(i) Electromeric effect and electrophilic attack.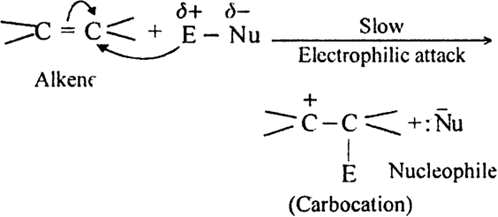
(ii) Attack of nucleophile. The nucleophile released in slow step combines with carbocation to give the addition product. 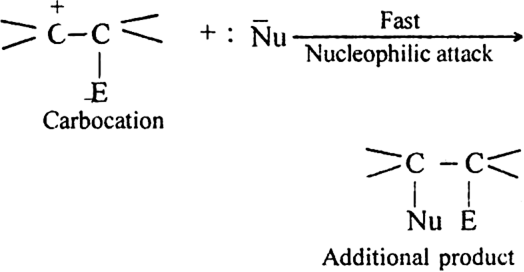
Discuss the mechanism of addition hydrogen acids to symmetrical alkenes. Justify the order of reactivity of halogen acids HI > HBr > HCl.
