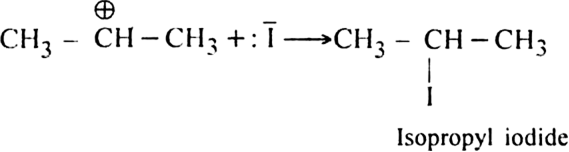Short Answer Type
Short Answer TypeWhat happens when:
(i) Ethyl alcohol is heated in the presence of Al2O3 at 493 K?
(ii) Ethylene dibromide is heated with zinc dust?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
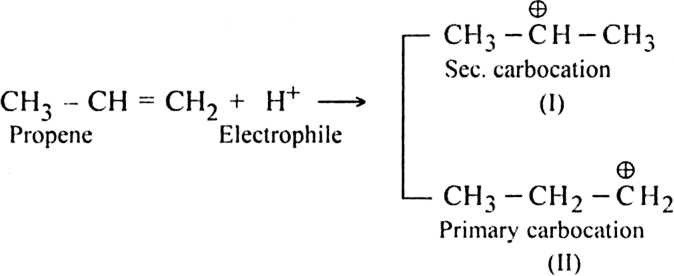
Long Answer TypeDiscuss the mechanism of addition hydrogen acids to symmetrical alkenes. Justify the order of reactivity of halogen acids HI > HBr > HCl.
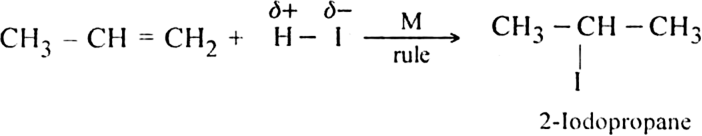
 of the HI to form isopropyl iodide as the main product.
of the HI to form isopropyl iodide as the main product.