 Long Answer Type
Long Answer TypeDiscuss the free radical mechanism of addition of HBr to propene.
Or
Give the mechanism of addition of HBr to propylene in the presence of peroxide.
The addition of HBr to propene yields 2-broniopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
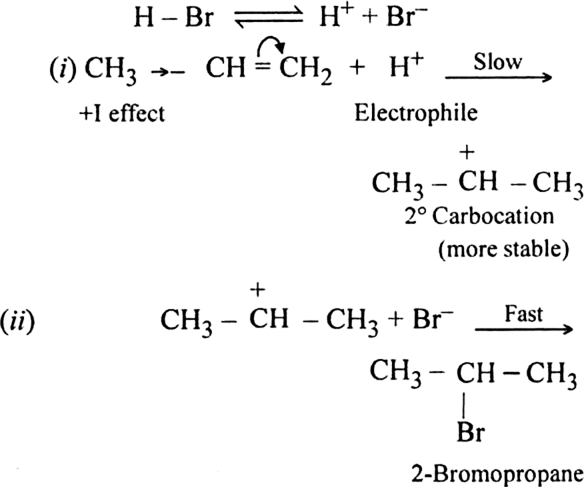
Formation of 1-Bromopropane:
In the presence of benzoyl peroxide, the addition of HBr to propene involves free radical mechanism in which Br-free radical is obtained by the action of benzoyl peroxide on HBr.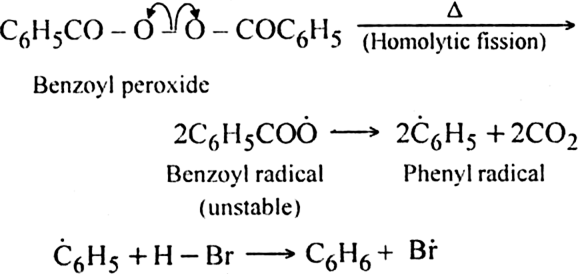
(i) Br radical adds to propene to form the more stable 2° free radical.
(ii) Free radical thus obtained rapidly abstracts a hydrogen atom from HBr to form 1-bromopropane.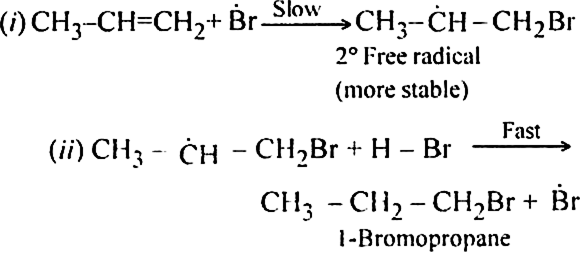
 Short Answer Type
Short Answer TypeWhy peroxide effect is shown only by HBr and not by HCl or HI?
Or
Explain why Kharasch effect is shown by HBr only and not by HCl or HI.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWrite IUPAC names of the products obtained by the ozonolysis of the following compounds:
Pent - 2-ene
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
3, 4-Dimethlhept-3-ene
