 Long Answer Type
Long Answer TypeGive two reactions of electrophilic addition on acetylene along with their mechanism.
(i) The addition of halogen: Chlorine and bromine add on to acetylene to form addition product. ![]()
Mechanism: It involves electrophilic addition and consists of the following steps: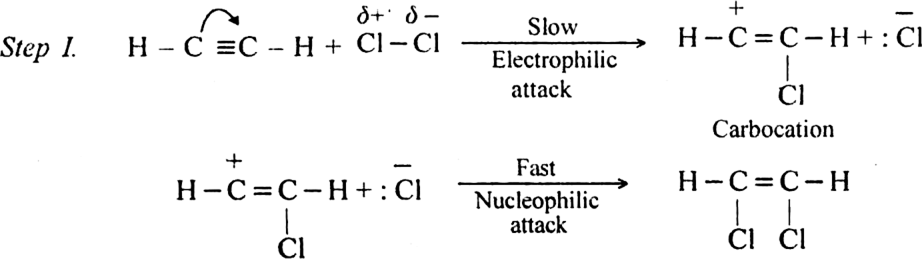
Step II. The electrophilic addition is repeated again when the final compound gets formed.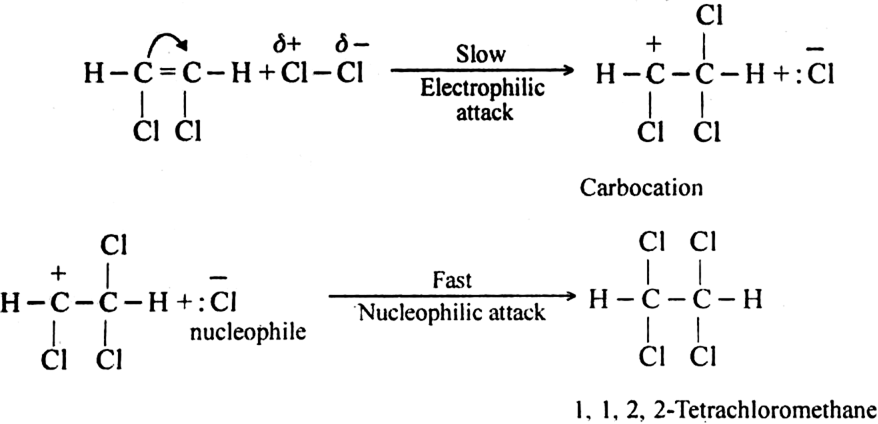
(ii) The addition of hydrogen halides. The hydrogen halides (HCl, HBr or HI) add-on triple bond to give rise to haloalkene or haloalkane.![]()
Mechanism: It involves electrophilic addition and consists of the following steps: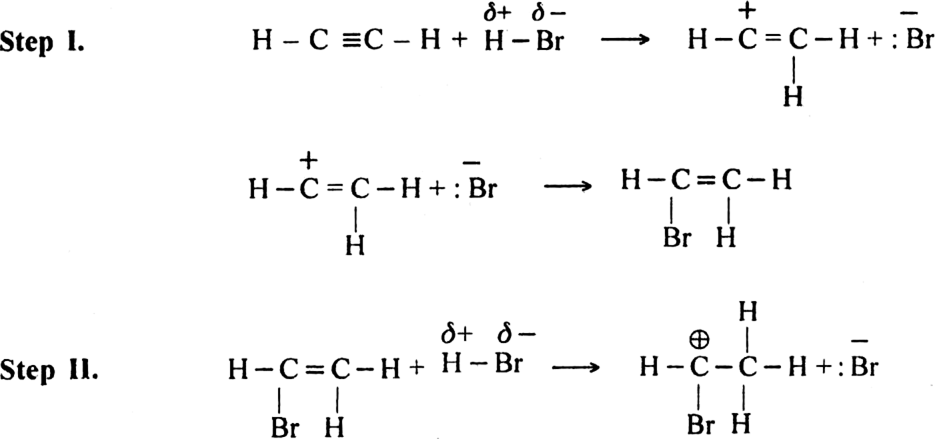
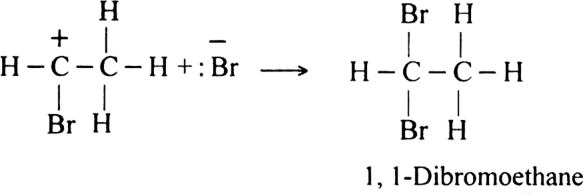
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeStarting from acetylene how will you obtain:
(i) Ethylidene iodide
(ii) Dichloro acetaldehyde
(iii) Ethylene?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeStarting from Ethyne, how will you prepare (No mechanism):
(i) Ethanol (ii) Vinyl chloride (iii) Methyl vinyl ether (iv) Vinyl acetate?
