 Long Answer Type
Long Answer TypeGive two reactions of electrophilic addition on acetylene along with their mechanism.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeStarting from acetylene how will you obtain:
(i) Ethylidene iodide
(ii) Dichloro acetaldehyde
(iii) Ethylene?
 -cloud.
-cloud.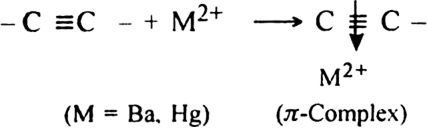
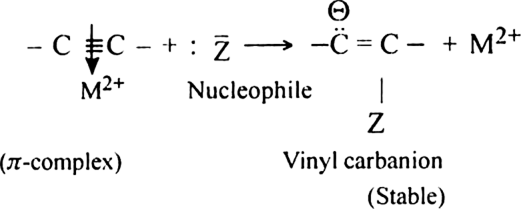
 -complex formation is responsible for the nucleophilic addition reaction of alkynes. For example, alkynes undergo following nucleophilic addition reactions:
-complex formation is responsible for the nucleophilic addition reaction of alkynes. For example, alkynes undergo following nucleophilic addition reactions:(i) The addition of water in the presence of dilute H2SO4 and HgSO4.
(ii) The addition of HCN in the presence of Ba(CN)2.
(iii) The addition of acetic acid in the presence of Hg2+ ion.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeStarting from Ethyne, how will you prepare (No mechanism):
(i) Ethanol (ii) Vinyl chloride (iii) Methyl vinyl ether (iv) Vinyl acetate?
