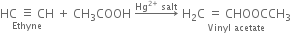Long Answer Type
Long Answer TypeGive two reactions of electrophilic addition on acetylene along with their mechanism.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeStarting from acetylene how will you obtain:
(i) Ethylidene iodide
(ii) Dichloro acetaldehyde
(iii) Ethylene?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeStarting from Ethyne, how will you prepare (No mechanism):
(i) Ethanol (ii) Vinyl chloride (iii) Methyl vinyl ether (iv) Vinyl acetate?
(i) Ethanol: It is obtained by the following steps of reaction: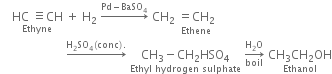
(ii) Vinyl chloride: Ethyne reacts with one mole of HCl to form vinyl chloride.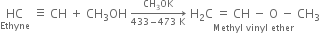
(iii) Methyl vinyl ether: When ethyne is passed into methyl alcohol at 433-473 K in the presence of small amount of potassium methoxide and under pressure, methyl vinyl ether is formed.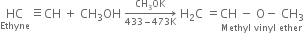
(iv) Vinyl acetate: Acetic acid adds on a molecule of ethyne in the presence of mercury salt to form vinyl acetate.