 Short Answer Type
Short Answer TypeYou are given samples of ethane, ethene and ethyne in three different containers. How will you distinguish them?
 Long Answer Type
Long Answer Type -electrons).
-electrons).
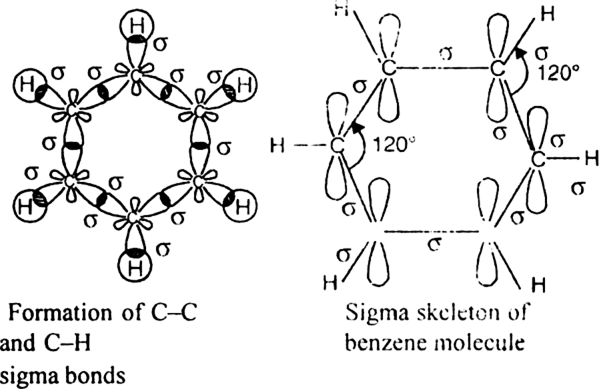
 -bonds. Since 2pz orbital on any carbon atom can overlap sideways with the 2pz orbital on adjacent carbon atom on either side equally well, a continuous
-bonds. Since 2pz orbital on any carbon atom can overlap sideways with the 2pz orbital on adjacent carbon atom on either side equally well, a continuous 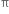 -molecular 3 orbitals will result which embraces all the six p-electrons as shown:
-molecular 3 orbitals will result which embraces all the six p-electrons as shown: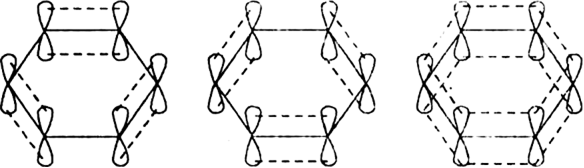
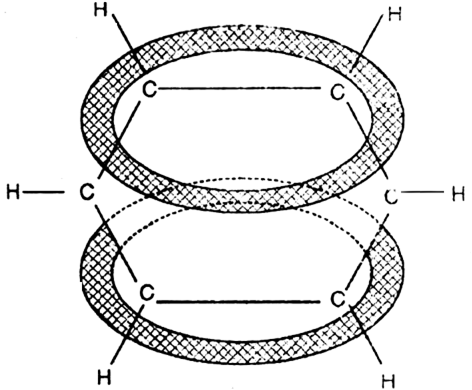
 Short Answer Type
Short Answer TypeBenzene ring has three double bonds in it but is still quite stable. Explain.
Or
Why is benzene extra-ordinary stable though it contains three double bonds?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type