 Long Answer Type
Long Answer TypeWhat are the characteristics of a compound to be aromatic?
Or
What do you mean by aromaticity?
Or
What are the necessary conditions for any system to be aromatic?
Write a short note on annulenes.
Annulenes are monocyclic conjugated polyenes which contain an even number of carbon atoms in their molecules. These are represented by the general formula (-CH = CH-)n where n = 2, 3, 4.....
Aromatic nature in annulenes: According to Huckel’s rule, annulenes containing (4n + 2) electrons and having a coplanar cyclic carbon skeleton should be aromatic in nature. For example,
(i) [4] Annulene. It is non-aromatic because it does not contain 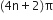 electrons.
electrons.
(ii) [6] Annulene. It is aromatic because it contains 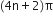 electrons.
electrons.
(iii) [8] Annulene. It is non-aromatic because it does not contain  electrons.
electrons.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow would you convert the following compounds to benzene:
(i) Benzoic acid
(ii) Benzene diazonium chloride?
