 Long Answer Type
Long Answer Type
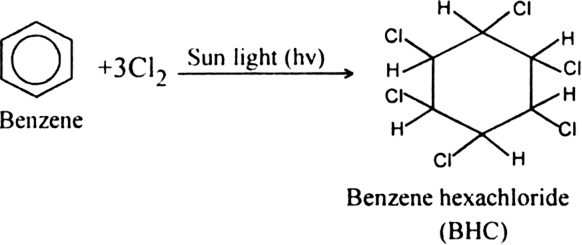
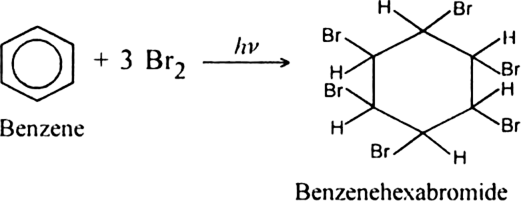
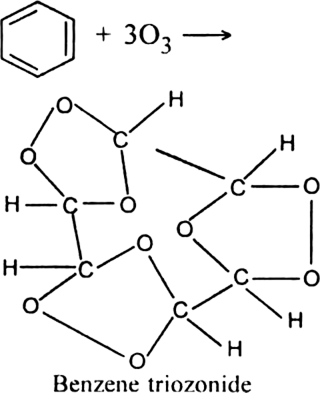
 Short Answer Type
Short Answer TypeWrite chemical equations for the combustion reaction of the following hydrocarbons:
Butane
Write chemical equations for the combustion reaction of the following hydrocarbons:
Pentene
Write chemical equations for the combustion reaction of the following hydrocarbons:
Hexyne
Write chemical equations for the combustion reaction of the following hydrocarbons:
Toluene
Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure for benzene?
