 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeOut of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
How will you explain the directive influence of alkyl group (in the case of toluene)?
How will you explain the directive influence of halogens?
Halogens (Cl, Br, l) contain three pairs of electrons and thus release electrons to the aromatic ring through resonance. But due to its electrons withdrawing (-Inductive effect) nature, it also intensifies the positive charge on carbocation. Thus, inductive effect and resonance effect work in the opposite direction and the result of two opposing effects is electron withdrawal. That is why halogens deactivate the aromatic ring for electrophilic substitution. Consider chloro-benzene.+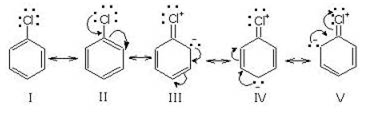
Thus chlorine atom deactivates the ring due to -I effect and directs the incoming electrophile to attack at the ortho and para position due to resonance effect.
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile E+:
(a) Chlorobenzene; 2, 4-dinitrochlorobenzene, p- nitro chlorobenzene
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss measures for the control of pollution problems with special reference to the use of C.N.G.
