 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeOut of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
How will you explain the directive influence of alkyl group (in the case of toluene)?
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile E+:
(a) Chlorobenzene; 2, 4-dinitrochlorobenzene, p- nitro chlorobenzene
(a) 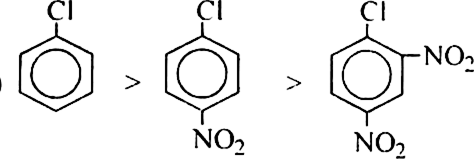
Nitro (NO2) group is a deactivating group. Its presence on the benzene ring will deactivate it towards electrophile attack since electrophile seeks a centre of high electron density. Thus, more the number of nitro groups present, lesser will be the reactivity of the compounds towards electrophilic substitution.
(b) Here, CH3 group is electron donating but NO2 group is electron withdrawing. Therefore, the maximum electron density will be in toluene, followed by p-nitrotoluene followed by p-dinitrobenzene. Thus, the overall reactivity decrease in the order: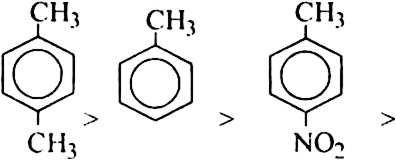
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss measures for the control of pollution problems with special reference to the use of C.N.G.
