 Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of the compound is
pent-4-yn-2-ene
pent-3-en-1-yne
pent-2-en-4-yne
pent-1-yn-3-ene
Consider the reactions,
The mechanisms of reactions (i) and (ii) are respectively
SN 1 and SN2
SN 1 and SN1
SN 2 and SN 2
SN 2 and SN1
A.
SN 1 and SN2
C2H5OH being a weaker nucleophile, when used as a solvent in case of hindered 1 ° halide, favours SN 1 mechanism while C2H5O- being a strong nucleophile in this reaction favours SN 2 mechanism.
CH3CH2Br undergoes Wurtz reaction. We may expect some of the following product
A: CH3CH2CH2CH3
B:
C: CH3-CH3
Select correct product.
Only A
A and B
A, B and C
A and C
In which of the below reaction do we find unsaturated carbonyl compounds undergoing a ring closure reaction with conjugated dienes?
Perkin reaction
Diels-Alder reaction
Claisen rearrangement
Hofmann reaction
IUPAC name of the compound
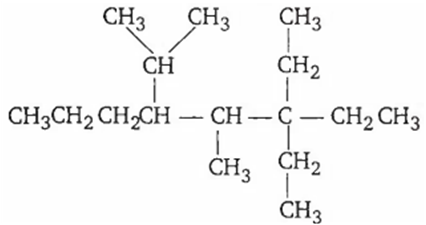
5-methyl-4-iso propyl-6, 6' diethyloctane
3,3-dimethyl, 3-ethyl-5-isopropyl octane
3,3-diethyl-4-methyl-5-(1,1-dimethyl) octane
3,3-diethyl-4-methyl-5-(1'-methylethyl) octane
Consider the following carbocations,
The correct sequence of the stability of these is
II < I < III < IV
II < III < I < IV
III < I < II < IV
IV < III < I < II
