 Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of the compound is
pent-4-yn-2-ene
pent-3-en-1-yne
pent-2-en-4-yne
pent-1-yn-3-ene
Consider the reactions,
The mechanisms of reactions (i) and (ii) are respectively
SN 1 and SN2
SN 1 and SN1
SN 2 and SN 2
SN 2 and SN1
CH3CH2Br undergoes Wurtz reaction. We may expect some of the following product
A: CH3CH2CH2CH3
B:
C: CH3-CH3
Select correct product.
Only A
A and B
A, B and C
A and C
Identify the alkyne in the following sequence of reactions,
A.
In Wacker process, alkene is oxidised into aldehyde.
Since on ozonolysis, only alkenes produce aldehydes, 'A' must be an alkene. To decide the structure of alkene that undergoes ozonolysis, bring the products together in such a way that O atoms are face to face and, replace O by double (=) bond. Thus,

In which of the below reaction do we find unsaturated carbonyl compounds undergoing a ring closure reaction with conjugated dienes?
Perkin reaction
Diels-Alder reaction
Claisen rearrangement
Hofmann reaction
IUPAC name of the compound
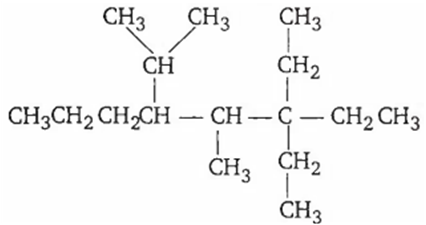
5-methyl-4-iso propyl-6, 6' diethyloctane
3,3-dimethyl, 3-ethyl-5-isopropyl octane
3,3-diethyl-4-methyl-5-(1,1-dimethyl) octane
3,3-diethyl-4-methyl-5-(1'-methylethyl) octane
Consider the following carbocations,
The correct sequence of the stability of these is
II < I < III < IV
II < III < I < IV
III < I < II < IV
IV < III < I < II
