 Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of the compound is
pent-4-yn-2-ene
pent-3-en-1-yne
pent-2-en-4-yne
pent-1-yn-3-ene
Consider the reactions,
The mechanisms of reactions (i) and (ii) are respectively
SN 1 and SN2
SN 1 and SN1
SN 2 and SN 2
SN 2 and SN1
CH3CH2Br undergoes Wurtz reaction. We may expect some of the following product
A: CH3CH2CH2CH3
B:
C: CH3-CH3
Select correct product.
Only A
A and B
A, B and C
A and C
CH3CH2NO2
CH3CH2NO2 + CH3NO2
2CH3NO2
CH2 CH2
B.
CH3CH2NO2 + CH3NO2
Under certain conditions, alkanes react with HNO3, a hydrogen atom being replaced by a nitre group (NO2). This process is known as nitration. Nitration of alkane may be carried out in the vapour phase between 150° to 475°C. Where upon a mixture of mono nitro alkanes is obtained.
Example : Ethane give a mixture of nitroethane and nitromethane.
During nitration chain fission of alkanes also takes place, so CH3NO2 is also obtained along with CH3CH2NO2.
In which of the below reaction do we find unsaturated carbonyl compounds undergoing a ring closure reaction with conjugated dienes?
Perkin reaction
Diels-Alder reaction
Claisen rearrangement
Hofmann reaction
IUPAC name of the compound
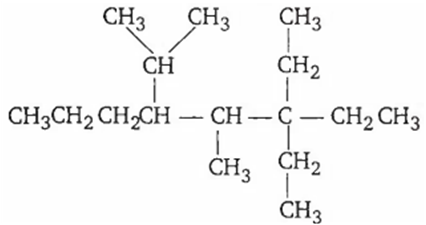
5-methyl-4-iso propyl-6, 6' diethyloctane
3,3-dimethyl, 3-ethyl-5-isopropyl octane
3,3-diethyl-4-methyl-5-(1,1-dimethyl) octane
3,3-diethyl-4-methyl-5-(1'-methylethyl) octane
Consider the following carbocations,
The correct sequence of the stability of these is
II < I < III < IV
II < III < I < IV
III < I < II < IV
IV < III < I < II
