 Multiple Choice Questions
Multiple Choice QuestionsThe reaction
CH2 = CH2 + H2 CH3-CH3 is called
Wurtz reaction
Kolbe's reaction
Sabatier-Senderens reaction
Carbylamine reaction
Formation of 2-butene as major product by dehydation of 2-butanol is according to
Markownikoff's rule
Saytzeff's rule
Peroxide effect
Anti-Markownikoffs rule
Acetic acid dissolved in benzene shows molecular weight
60
120
180
240
B.
120
Acetic acid when dissolved in benzene exist as doubly associated molecules in benzene due to hydrogen bonding.
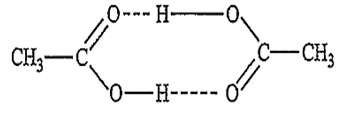
As a result, the experimentally observed value of the colligative properties is lower and the molecular mass is double than the expected value.
Ethene gives with acidic KMnO4 solution
ethylene glycol
ethylene oxide
formaldehyde
acetaldehyde
An organic alkadiene on reductive ozonolysis produces
(i) acetaldehyde
(ii) acetone
(iii) 2-methylpropane-1,3-dial
The formula of alkadiene will be
CH3C(CH3)=CHCH(CH3)CH=CHCH3
CH3CH(CH3)CH=C(CH3)CH=CHCH3
CH3C(CH3)=CHCHC(CH3)=CHCH3
CH3CH2CH(CH3)CH=CHC(CH3)=CH2
2-chloro-3-methylbutane is treated with sodium in etherial solution, then it will give
2,4-dimethylhexane
3,5-dimethylhexane
2,3,4,5-tetramethylhexane
2,6-dimethyloctane
