 Short Answer Type
Short Answer Type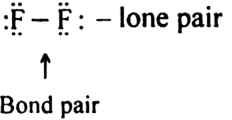
 Long Answer Type
Long Answer TypeDescribe the industrial uses of hydrogen which depend on:
(i) the heat liberated when it burns,
(ii) its ability to react with vegetable oil in the presence of catalyst,
(iii) its ability to unite with nitrogen.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type