 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat properties of water make it useful as a solvent? What type of compounds can it: (i) dissolve and (ii) hydrolyse?
 Short Answer Type
Short Answer Type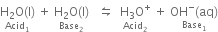
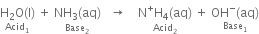
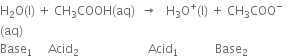
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat are the ways in which water molecules are bound to an anhydrous salt to form a hydrate?
