 Short Answer Type
Short Answer TypeWhen a piece of calcium metal is dropped into a beaker of cold water, a vigorous reaction takes place and the metal gradually disappears to form a white suspension.
(i) Write down the equation for the reaction.
(ii) Why does the solution become milky?
(iii) On filtering, a clear solution is obtained. What is the name of the clear solution?
What is the importance of heavy water with regard to nuclear power generation?
Or
Discuss the importance of heavy water in nuclear reactor.
Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes?
 Long Answer Type
Long Answer TypeHydrogen peroxide can be prepared on a large scale by any of the following methods:
1. Electrolytic process. In this process, the electrolysis of 50% sulphuric acid is carried out at low temperature using platinum electrodes and a current of high density. Persulphuric acid is formed.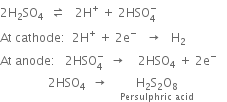
Persulphuric acid is distilled. Hydrolysis occurs and a distillate containing about 30% H2O2 is obtained.
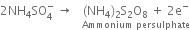
Modification. Recently, it has been observed that if instead of 50% H2SO4, an equimolar mixture of H2SO4 and ammonium sulphate is electrolyzed, a more concentrated solution of H2O2 is obtained.
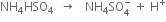
At anode:
At cathode: 
Ammonium persulphate formed around anode is withdrawn and heated at 43 mm pressure when its hydrolysis occurs forming hydrogen peroxide.
Hydrogen peroxide along with water distills over. Ammonium bisulphate can be used again. This method gives 30-40% aqueous solution of hydrogen peroxide.
2. From 2-ethylanthraquinone. This is the most recent process for the manufacture of hydrogen peroxide. 2-ethyl anthraquinone is catalytically reduced to 2-ethylanthraqinol in an organic solvent by passing hydrogen gas in the presence of palladium (catalyst). The reduced product i.e. 2-ethyl anthraquinol is dissolved in a mixture of benzene and cyclohexanol. On passing air through it, 2-ethylanthraquinone and hydrogen peroxide are formed.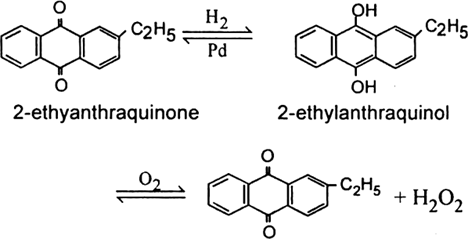
H2O2 is extracted with water to give a 20% solution. 2-ethyl anthraquinone is reformed which is again used. The method, thus, involves alternate oxidation and reduction steps.
 Short Answer Type
Short Answer TypeWhat precautions are necessary while storing hydrogen peroxide?
Or
Explain why hydrogen peroxide is stored in coloured/plastic bottles.
