 Multiple Choice Questions
Multiple Choice QuestionsDeuterium oxide is used in nuclear reactor as
source of -particle
source of deuteron
moderator
fuel
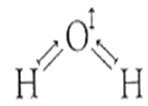
H2O is polar, whereas BeF2 is not because
electronegativity of F is greaterthan that of O.
H2O involves H-bonding, whereas BeF2 is a discrete molecule
H2O is angular and BeF2 is linear
H2O is linear and BeF2 is angular.
C.
H2O is angular and BeF2 is linear
Because of linear shape, dipole moments cancel each other in BeF2 (F Be F) and thus, it is non-polar, whereas H2O is V-shaped and hence, it is polar.
The correct order of solubility ofthe following compounds in water is
Ba(OH)2 < Mg(OH)2
BaCO3 > CaCO3
Ca(OH)2 Mg(OH)2
CaSO4 < MgSO4
Assertion : H2O2 has higher boiling point than water.
Reason : H2O2 has stronger dipole-dipole interactions than that shown by water.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion: Permanent hardness of water is removed by treatment with washing soda.
Reason: Washing soda reacts with soluble calcium and magnesium chlorides and sulphates in hard water to form insoluble carbonates.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Which of the following is used as moderator :
Hard water
Heavy water
Non-ionised water
Mineral water
