 Short Answer Type
Short Answer TypeWrite the monomers used for getting the following polymers.
(i) Polyvinyl chloride (ii) Teflon (iii) Bakelite.
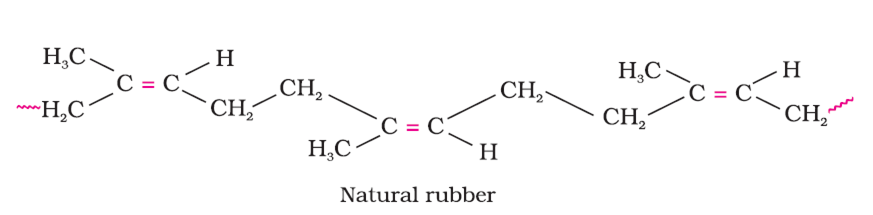
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Write the names and structures of the monomers of the following polymers:
(1) Buna-S (2) Buna-N (3) Dacron (4)Neoprene.
What is biodegradable polymer and give an example of a biodegradable aliphatic polyester?
