 Short Answer Type
Short Answer TypeWrite the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Glyptal
(iii) Polyvinyl chloride
What is a biodegradable polymer? Give an example of biodegradable aliphatic polyester.
Differentiate between thermoplastic and thermosetting polymers. Give one example of each.
(i) What is the role of t-butyl peroxide in the polymerization of ethene?
(ii) Identify the monomers in the following polymer:
OR
Write the mechanism of free radical polymerization of ethene.
(i) In polymerisation of ethene, the source of free radical is needed to initiate the chain reaction. Such free radicals are usually produced by the decomposition of peroxides like t- butyl peroxide or benzoyl peroxide.
(ii)The given polymer is nylon 6, 6.
Monomers of nylon 6,6 are adipic acid (HOOC(CH2)4COOH)and hexamethylenediamine (H2N(CH2)6NH2).
(iii)Elastomers of rubbers have the weakest intermolecular forces of attraction, while fibres have the strongest intermolecular forces of attraction. Plastics have intermediate forces of attraction.
The increasing order of intermolecular forces of attraction of the given polymers is as follows:
Terylene> Polystyrene > Buna-S
Or
The mechanism of free radical polymerization of ethene.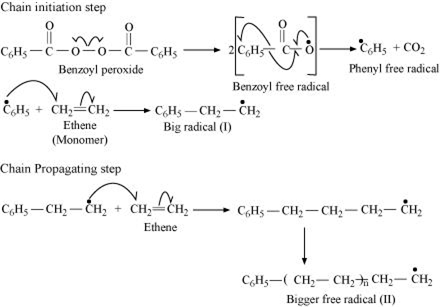
i) What is the role of sulphur in the vulcanization of rubber?
ii) Identify the monomers in the following polymer: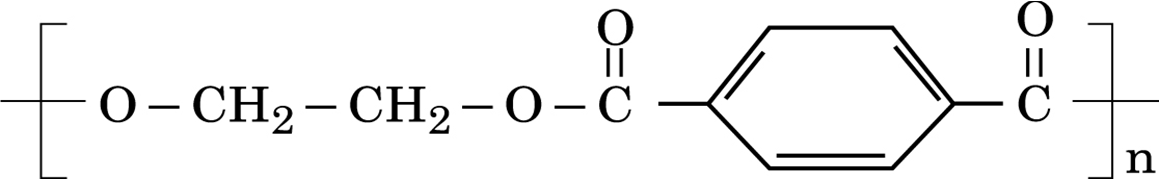
iii)Arrange the following polymers in the increasing order of their intermolecular forces:
Terylene, polythene, Neoprene
Write the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Dacron
(iii) Neoprene