 Multiple Choice Questions
Multiple Choice QuestionsIf is the weight average molecular weight and is the number average molecular weight of a polymer, the poly disparity index (PDI) of the polymer is given by
If the number average molecular weight and weight average molecular weight of a polymer are 40000 and 60000 respectively, the polydispersity index of the polymer will be
>1
<1
1
zero
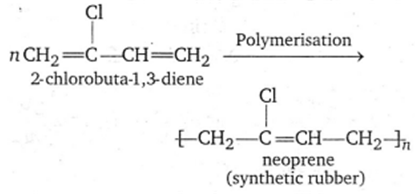
The monomer of neoprene is
1,3-butadiene
2-chloro-1,3-butadiene
2-methyl-1,3-butadiene
vinyl chloride
B.
2-chloro-1,3-butadiene
Chloroprene (or 2-chlorobuta-1,3-diene) is the monomer of synthetic rubber, neoprene.
Example of a biodegradable polymer pair is
nylon-6, 6 and terylene
PHBV and dextron
bakelite and PVC
PET and polyethylene
The polymer obtained with methylene bridges by condensation polymer is
PVC
buna-S
polyacrylonitrile
bakelite
Observe the following polymers.
I. PHBV
II. Nylon-2-nylon-6
III. Glyptal
IV. Bakelite
Biodegradable polymer(s) from the above is/ are
III
I and II
IV
III and IV
