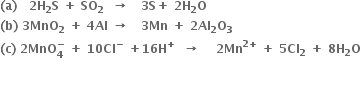Long Answer Type
Long Answer TypeOxidation: Oxidation is defined as a chemical reaction in which oxidation number of an element increases.
Reduction: Reduction is defined as a chemical reaction in which oxidation number of an element decreases.
Oxidising agent: Oxidising agent is a species which undergoes a decrease in oxidation number.
Reducing agent: Reducing agent is a species which undergoes an increase in oxidation number.
For example in the reaction:
The oxidation number of S increases from -2 to 0 while the oxidation number of Fe decreases from +3 (in FeCl3) to +2 (in FeCl2). Therefore H2S is oxidised and FeCl3 is reduced. Thus FeCl3 acts as an oxidising agent and H2S acts as a reducing agent.
 Short Answer Type
Short Answer TypeConsider the elements:
Cs, Ne, I and F.
(a) Identify the element that exhibits only negative oxidation state.
(b) Identify the element that exhibits only positive oxidation state.
(c) Identify the element that exhibits both positive and negative oxidation states.
(d) Identify the element which exhibits neither negative nor positive oxidation state.
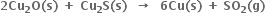
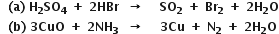
 Long Answer Type
Long Answer Type(b) HCHO(l) + 2[Ag (NH3)2]+ (aq) + 3OH-(aq) → 2Ag(s) + HCOO–(aq) + 4NH3(aq) + 2H2O(l)
(c) HCHO (l) + 2 Cu2+ (aq) + 5 OH–(aq) → Cu2O(s) + HCOO-(aq) + 3H2O(l)
(d) N2H4(l) + 2H2O2(l) → N2(g) + 4H2O(l)
(e) Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)