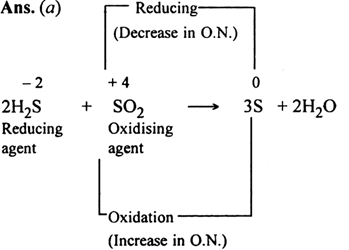
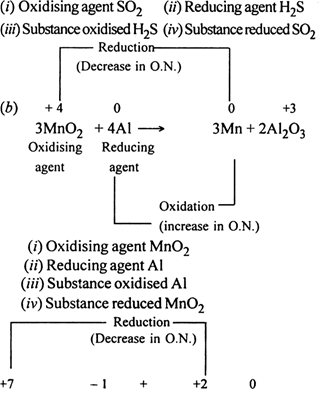
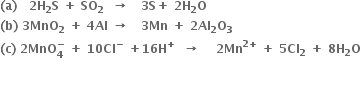Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeConsider the elements:
Cs, Ne, I and F.
(a) Identify the element that exhibits only negative oxidation state.
(b) Identify the element that exhibits only positive oxidation state.
(c) Identify the element that exhibits both positive and negative oxidation states.
(d) Identify the element which exhibits neither negative nor positive oxidation state.
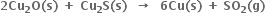
 Long Answer Type
Long Answer Type(b) HCHO(l) + 2[Ag (NH3)2]+ (aq) + 3OH-(aq) → 2Ag(s) + HCOO–(aq) + 4NH3(aq) + 2H2O(l)
(c) HCHO (l) + 2 Cu2+ (aq) + 5 OH–(aq) → Cu2O(s) + HCOO-(aq) + 3H2O(l)
(d) N2H4(l) + 2H2O2(l) → N2(g) + 4H2O(l)
(e) Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)