 Short Answer Type
Short Answer TypeWhat are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?  
 
 Long Answer Type
Long Answer TypeCalculate the oxidation number of sulphur, chromium and nitrogen in H2SO5,   and 
 and   Suggest structure of these compounds. Count for the fallacy.
 Suggest structure of these compounds. Count for the fallacy.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeCalculate the oxidation number of:
(i) Cr in dichromate ion
(ii) Mn in manganate ion
(iii) S in tetrathionate ion.
Compute the oxidation number of:
(i) Ni in |Ni(Fe(C2O4)3|
(ii) Fe in K3|Fe(C2O4)3|
(i) Let the oxidation number of Ni = x
Writing the oxidation number of each atom at the top of its symbol. 
                          x       0
                         [Ni  (CO)4]
The algebraic sum of the oxidation number of various atoms = 0
                  x + 4(0) = 0
                          x = 0
(ii) Let the oxidation number of Fe = x
Writing the oxidation number of each atom at the top of its symbol,
                        +1                  x - 2
                         K3               [Fe(C2O4)3]
The algebraic sum of the oxidation number of various atoms = 0
          3(+1) + x + 3(-2) = 0
                  3 + x - 6 = 0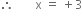
 Short Answer Type
Short Answer Type