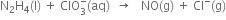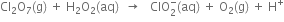Long Answer Type
Long Answer Type
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
The Mn3+ ions is unstable in solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write a balanced ionic equation for the reaction.
The skeleton equation is
Mn3+ (aq) → Mn2+ (aq) + MnO2(s) + H+(aq)
Let us balance the above equation by ion electron method.
1. Write the oxidation and reduction half-reactions by observing the changes in oxidation number and writing these separately
Oxidation half-reaction:
+3 +4

Reduction half reaction:
+3 +2

2. Balancing the oxidation half reaction
(i) Add 1 electron towards R.H.S. to balance the charge on Mn.
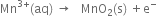
(ii) Balance the charges by adding four H+ towards R.H.S.

[Balanced oxidation half reaction]
(iii) Balance O atoms by adding two H2O molecules downwards L.H.S.

[Balanced oxidation half reaction]
3. Balancing the reduction half reaction:
Add 1 electron towards L.H.S. to balance the charge on Mn.
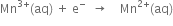
(Balanced reduction half reaction]
4. Adding balanced oxidation half reaction and balanced reduction half-reaction.

This is balanced redox equation for disproportionation reaction.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypePermanganate (VII) ion,  in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction.
in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction.
Balance the following equations in basic medium by ion-electron method and oxidation number method: