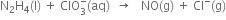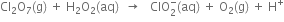Long Answer Type
Long Answer Type
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water. 
The Mn3+ ions is unstable in solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write a balanced ionic equation for the reaction.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type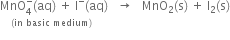
The skeleton equation is: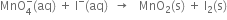
Separation of the equation in two half reactions
(i) Write the O.N. of the atoms involved in the equation
   -7 -2           -1                 +4 -2    0
     
 
(ii) Identify the atoms which undergo a change in O.N.
    +7        -1                    +4         0
(iii) Find out the species involved in the oxidation and reduction half-reactions: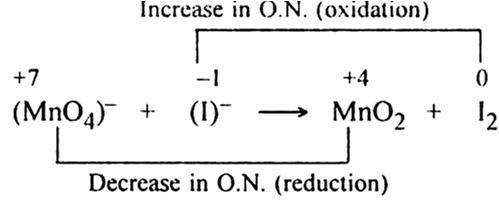
Oxidation half-reaction:
                   
Reduction half-reaction:
                
Balancing the oxidation half reaction
                    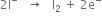
Balancing the reduction half reaction
 The reduction half-reaction is:
                       
(i) As the decrease in O.N. is 3, therefore add 3e- on the reactant side
                             
(ii) Balance O atoms by adding two H2O molecules on the product side 
               
(iii) Balance the charges, by adding four OH- on the product side and balance H atoms by adding four H2O molecules on the reactant side.        Thus, the reduction half-reaction is balanced. Adding the two half reactions. In order to equate the electrons, multiply equation. (i) by 3 and equation, (ii) by 2 and add the two equations.
      Thus, the reduction half-reaction is balanced. Adding the two half reactions. In order to equate the electrons, multiply equation. (i) by 3 and equation, (ii) by 2 and add the two equations.
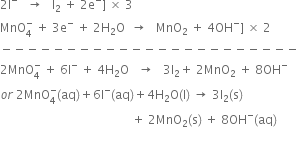
Permanganate (VII) ion,   in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction. 
 in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction. 
Balance the following equations in basic medium by ion-electron method and oxidation number method: