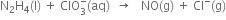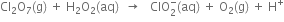Long Answer Type
Long Answer Type
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
The Mn3+ ions is unstable in solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write a balanced ionic equation for the reaction.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypePermanganate (VII) ion,  in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction.
in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction.
Balance the following equations in basic medium by ion-electron method and oxidation number method:
(a) Ion-electron method:
1. Write the oxidation and reduction half-reactions by observing the changes in oxidation numbers.
Oxidation half-reaction.
0 +1

Reduction half-reaction.
0 -3

2. Balancing the oxidation half reaction.
(i)Balancing P atoms by multiply  by 4
by 4

(ii) Add 4 electrons towards RHS to balance the charges.

(iii) Add 8OH- towards LHS to balance the charges

(Balanced oxidation half reaction)
3. Balancing the reduction half reaction
(i) Balance P atoms by multiplying PH3 by 4
0 -3
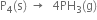
(ii) Add 12 electrons towards LHS to balance the charge on P

(iii) Balance oxygen and hydrogen atoms by adding 12H2O towards LHS and 12OH- towards
RHS
[Balanced reduction half reaction]
4. Multiply balanced oxidation half-reaction by 3 and add it to the balanced reduction half-reaction, we have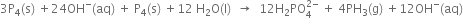
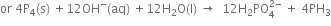

This is the balanced redox equation,
(b) Oxidation number method
(i) The skeleton equation along with oxidation number of each atom is
0 -3 + 1 +1 +1 -2
(ii) The oxidation number of P increases by 1 per atom while that of P decreases by 3 per atom.
P4 acts both as an oxidising as well as reducing agent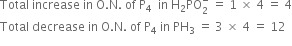


(iv) Balance O atoms by multiplying OH- by 6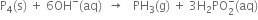
(v) Balance H atoms by adding three H2O towards L.H.S. and three OH- towards R.H.S.

This is the balanced equation.