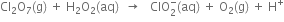Long Answer Type
Long Answer Type
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
The Mn3+ ions is unstable in solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write a balanced ionic equation for the reaction.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypePermanganate (VII) ion,  in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction.
in basic solution oxidises iodide ion, I- to produce molecule iodine (I2) and manganese (IV) oxide (MnO2). Write a balanced ionic equation to represent redox reaction.
Balance the following equations in basic medium by ion-electron method and oxidation number method:
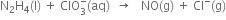
(A) Ion electron method:
1. Write the oxidation and reduction half reaction by observing the changes in oxidation number.
Oxidation half reaction:
-2 +2

+5 -1
Reduction half reaction: 
2. Balancing the oxidation half reaction:
(i) Balancing N atoms by multiplying NO by 2

(ii) Add eight electron towards R.H.S. to balance the charges

(iii) Add eight OH- ions towards LHS to balance the charge.

(iv) Balance O atoms by adding six H2O molecules towards R.H.S.

[Balanced oxidation half reactions]
3. Balancing the reduction half reaction:
(i) Add 6 electrons towards LHS to balance the charges on Cl atom

(ii) Add six OH- ions towards RHS to balance the charges

(iii) Balance O atoms by adding three H2O molecules towards LHS.
(iv) Multiplying balanced oxidation half reaction by 3 and reduction half reaction by 4 to equate the electrons and add both the half reactions.
This is the balanced redox equation.
(b) Oxidation number method
(i) The skeleton equation along with oxidation number of each atom is
-2 + 1 +5 +2 -2 -1
(ii) The oxidation number of N increase by 4 per N atom while that of CI decreases by 6 per atom.
Therefore,  acts as the reducing agent while
acts as the reducing agent while  acts as the oxidising agent.
acts as the oxidising agent.
(iii) Equalise the increase/decrease in O.N. by multiplying  and
and 
(iv) To balance N and Cl atoms, multiplying NO by 6 and Cl- by 4.
(v) Balance O atoms, by adding six H2O molecules towards R.H.S.

which is the balanced equation.