151.
In Ostwald’s process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.0 g of ammonia and 20.0 g of oxygen?
Mass of the ammonia = 17 g
Mass of Oxygen molecule= 32g
Mass of nitric oxide= 30g
Mass of water =18g
The reaction involved in the manufacturing process is:

4 x 17 = 68 g 5 x 32 = 160 g 4 x 30 = 120 g
From the available data:
68g of NH3 will react with O2 = 160 g
10g of NH3 will react with O2 = 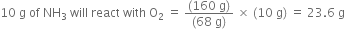
But oxygen which is actually available (20.0 g) is less than the amount which is needed. Therefore, oxygen is the limiting reactant.
Now, 160 g of O2 will form NO = 120 g
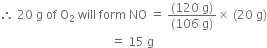
390 Views
 Short Answer Type
Short Answer Type
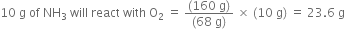
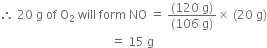
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type
