 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss in brief the working of an electrochemical cell.
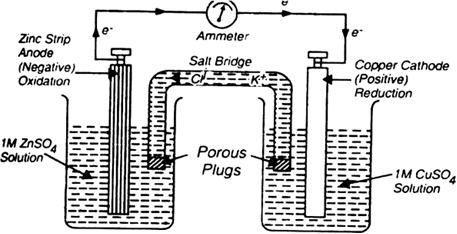
The ends of the salt-bridge are plugged with glass wool or cotton.
Reaction in a galvanic cell: When the two electrodes are connected through a copper wire, the reaction takes place and electric current begins to flow as shown by the ammeter. The following reactions occur at different electrodes:
At anode (Oxidation half-reaction):

At cathode (Reduction half reaction):

Oxidation takes place at anode and reduction takes place at the cathode. The electrons from the anode flow to the cathode. The anode is a negative electrode and cathode is a positive electrode. Zn2+ ions dissolve in the anode compartment while Cu2+ ions are deposited on the cathode. The neutrality of the two solutions is maintained by a salt bridge by providing cations and anions to replace the ions lost or produced in two half cells. The complete cell reaction is
In such cells, energy is liberated in the form of electrical energy.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeGive the points of difference between a redox reaction occurring in a beaker and in a cell ?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type
