 Multiple Choice Questions
Multiple Choice QuestionsPoint out the correct statement.
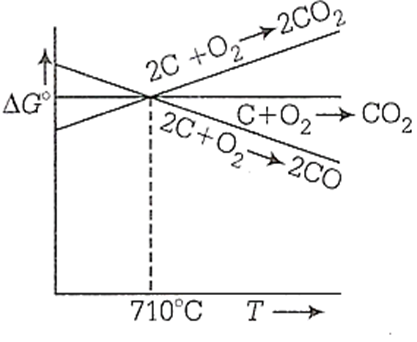
Below 710C, C is better reducing agent than CO
Below 710°C, CO is better reducing agent than C
Below 710C, CO is an oxidising agent
Below 710C, CO, is a reducing agent
B.
Below 710°C, CO is better reducing agent than C
Among all the statements given, statement b is correct.
Below 710C, CO is better reducing agent than CO. It can depicted by using the following graph
KMnO4 reacts with ferrous sulphate according to the following equation,
Mn + 5Fe2+ + 8H- Mn2+ + 2Fe3+ + 4H2O
Here, 10 mL of 0.1M KMnO4 is equivalent to
50 mL of 0.1 M FesO4
20 mL of 0.1 M FesO4
40 mL of 0.1 M FesO4
30 mL of 0.1 M FeSO4
Which of the following species do not show disproportionation on reaction?
ClO-, Cl , Cl and Cl
Cl
Cl
ClO-
None of these
For the reaction,
NH3 + OCl- N2H4 + Cl-
occurring in basic medium, the coefficient of N2H4 in the balanced equation will be
1
2
3
4
In an oxidation-reduction reaction, MnO ion is converted to Mn2+. What is the number of equivalents of KMnO4 (mol. wt.= 158) present in 250 mL of 0.04 M KMnO4 solution ?
0.02
0.05
0.04
0.07
Which of the following is wrong statement?
Ni(CO)4 has oxidation number +4 for Ni
Ni(CO)4 has zero oxidation number for Ni
Ni is metal
CO is gas
Consider a titration of potassium dichromate solution with acidified Mohr's salt solution using diphenyl amine as indicator. The number of moles of Mohr's Salt required per mole of dichromate is:
3
4
5
6
When MnO2 is fused with KOH, a colouredcompound is formed. The product and its colour is:
K2MnO4 , purple colour
Mn2O3 , brown
Mn2O4 , black
KaMnO4 , purple
