 Long Answer Type
Long Answer Type(a) Explain the following:
(i) Henry’s law about the dissolution of a gas in a liquid.
(ii) Boiling point elevation constant for a solvent.
(b) A solution of glycerol (C3H8O3) in water was prepared by dissolving some glycerol in 500 g of water. This solution has a boiling point of 100.42°C. What mass if glycerol was dissolved to make this solution? (Kb for water = 0.512 K kg mol-1)
a) Calculate the freezing point of the solution when 1.9 g of MgCl2 (M = 95 g mol−1) was dissolved in 50 g of water, assuming MgCl2 undergoes complete ionization.
(Kf for water = 1.86 K kg mol−1)
(b)
(i) Out of 1 M glucose and 2 M glucose, which one has a higher boiling point and why?
(ii) What happens when the external pressure applied becomes more than the osmotic pressure of solution?
OR
(a)When 2.56 g of sulphur was dissolved in 100 g of CS2, the freezing point lowered by 0.383 K. Calculate the formula of sulphur (Sx).
(Kf for CS2 = 3.83 K kg mol−1, Atomic mass of sulphur = 32 g mol−1]
(b)Blood cells are isotonic with 0.9% sodium chloride solution. What happens if we place blood cells in a solution containing
(i)1.2% sodium chloride solution?
(ii)0.4% sodium chloride solution?
 Short Answer Type
Short Answer Type(i) Gas (A) is more soluble in water than Gas (B) at the same temperature which one of two gases will the higher value of KH (Henry's constant) and why ?
(ii)In non-ideal solution, what type of deviation shows the formation of maximum boiling azeotropes?
Calculate the boiling point of the solution when 4g of MgSO4 (M =120 g mol-1) was dissolved in 100g of water,assuming MgSO4 undergoes complete ionization.
(Kb for water =0.52 K kg mol-1)
Differentiate between molarity and molality values for a solution. What is the effect of change in temperature on molarity and molality values?
A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 mL of water has an osmotic pressure of 0.335 torr at 25°C.
Assuming that the gene fragment is a non-electrolyte, calculate its molar mass.
 Long Answer Type
Long Answer Type(a) Define the following terms :
(i) Molarity
(ii) Molal elevation constant (Kb)
(b) A solution containing 15 g urea (molar mass = 60 g mol–1) per litre of the solution in water has the same osmotic pressure (isotonic) as a solution of glucose (molar mass = 180 g mol–1) in water. Calculate the mass of glucose present in one litre of its solution.
OR
(a) What type of deviation is shown by a mixture of ethanol and acetone? Give reason.
(b) A solution of glucose (molar mass = 180 g mol–1) in water is labelled as 10% (by mass). What would be the molality and molarity of the solution?
(Density of solution = 1.2 g mL–1)
(i) Molarity of a solution is defined as the number of moles of solute present in one litre of the solvent.
(ii) Molal elevation constant (Kb) is defined as the elevation of the boiling point of a solution when one mole of a non-volatile solute is dissolved in one kilogram of a volatile solvent.
(b) Osmotic pressure π = CRT = nVRT/2
where n is the number of moles of solute present in volume V of solvent.
It is given that the solution of urea is isotonic with the solution of glucose, thus
Or
a) A mixture of ethanol and acetone shows positive deviation from Raoult's Law. Pure ethanol possesses hydrogen bonding. The introduction of acetone between the molecules of ethanol results in breaking of some of these hydrogen bonds. Due to the weakening of interactions, the solution shows positive deviation from Raoult’s law.
(b)
10% by mass solution of glucose in water means that 10 g of glucose in present in 100 g of the solution i.e., 10 g of glucose is present in (100 - 10) g = 90 g of water.
Molar mass of glucose (C6H12O6) = 180 g mol-1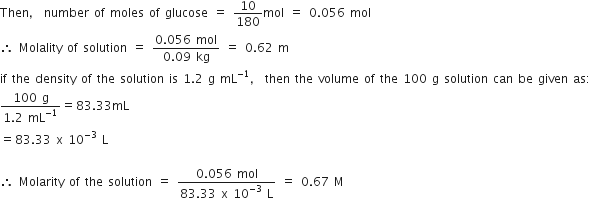
 Short Answer Type
Short Answer Type