 Multiple Choice Questions
Multiple Choice QuestionsA mixture of gases contains H2 and O2 gases in the ratio of 1:4 (w/w).what is the molar ratio of the two gases in the mixture?
1:4
4:1
16:1
16:1
The boiling point of 0.2 mol kg-1 solution of X in water is greater than the equimolal solution of Y in water. Which one of the following statements is true is this case?
X is undergoing dissociation in water
The molecular mass of X is greater than the molecular mass of Y.
Molecular mass of X is less than the molecular mass of Y,
Molecular mass of X is less than the molecular mass of Y,
Which one of the following electrolytes has the same value of van't Hoff's factor (i) as that of Al2(SO4)3 (if all are 100% ionised)?
K2SO4
K3[Fe(CN)6]
Al(NO3)3
Al(NO3)3
In Duma's method for estimation of nitrogen 0.25 g of an organic compound gave 40 ml of nitrogen collected at 300 K temperature and 725 mm pressure. If the aqueous tension at 300 K is 25 mm the percentage of nitrogen in the compounds is,
17.36
18.20
16.76
15.76
Of the following 0.10 m aqueous solutions, which one will exhibit the largest freezing point depression?
KCl
C6H12O6
Al2(SO4)3
Al2(SO4)3
The weight of silver (at. wt. = 108) displaced by a quantity of electricity which displaces 5600 mL of O2 at STP will be
5.4 g
10.8 g
54.0 g
54.0 g
Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25oC are 200 mmHg and 41.5 mmHg respectively, Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be
(molecular mass of CHCl3= 119.5 u and molecular mass of CH2Cl2 = 85 u)
173.9 mmHg
615.0 mmHg
347.9 mmHg
347.9 mmHg
The freezing point depression constant for water is -1 . 86o C m-1. If 5.00 g Na2SO4 is dissolved in 45.0 g H2O, the freezing points is changed by -3.82oC. Calculate the van't Hoff factor for Na2SO4.
2.63
3.11
0.381
0.381
A.
2.63
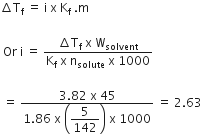
The van't Hoff factor, i for a compound which undergoes dissociation in one solvent and association in other solvent is respectively.
Less than one and less than one
Greater than one and less than one
Greater than one and greater than one
Greater than one and greater than one
A 0.1 molal aqueous solution of a weak acid is 30% ionised. if Kf for water is 1.86oC/m, the freezing point of the solution will be
-18oC
-0.54oC
-0.36oC
-0.36oC
