 Short Answer Type
Short Answer TypeThe vapour density of a gas A is four times that of B. If the molecular mass of B is M what is the molecular mass of A?
According to the relationship:
Mass=2 x Vapour density
we have give mass of B is M, thus Vapour density of B is,
V.D.=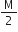
also, given A is four times that of B, then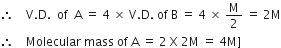
In a certain compound, the percentage of an element (At. mass 12) is 92.3. What is its mole ratio?
How many litres of oxygen at S.T.P. will be required to burn completely 2.2g of propane?
A substance has empirical formula CH2O, its vapour density is 30. What is its molecular formula?
