 Short Answer Type
Short Answer TypeThe vapour density of a gas A is four times that of B. If the molecular mass of B is M what is the molecular mass of A?
In a certain compound, the percentage of an element (At. mass 12) is 92.3. What is its mole ratio?
As, we have given the relative number of A =75.8%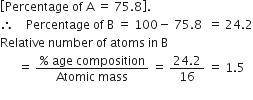
Hence, the relative number of B = 1.5%
How many litres of oxygen at S.T.P. will be required to burn completely 2.2g of propane?
A substance has empirical formula CH2O, its vapour density is 30. What is its molecular formula?
