 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeClassify the following as pure substances or mixtures: air, milk, graphite, diamond, gasoline, tap water, distilled water, oxygen, one rupee coin, 22 carat gold, steel, iron, sodium chloride, iodized table salt.
Separate the following substances into elements and compounds: Graphite, Diamond, Distilled water, Oxygen, Iron, Sodium chloride, Blue vitriol.
How will you separate the following mixtures?
(i) Oil and water
(ii) Iron-filings and saw dust.
(iii) Glass powder and sugar.
 Long Answer Type
Long Answer TypeThe reaction is 
Total mass of the reactants
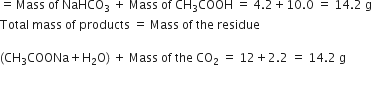
Thus, total mass of reactants (14.2) g
= Total mass of products (14.2) g
This illustrates the law of conservation of mass.
 Short Answer Type
Short Answer TypeIf 6.3g of NaHCO3 are added to 15.0 g of CH3COOH solution, the residue is found to weigh 18.0g. What is the mass of CO2 released in the reaction?
 Long Answer Type
Long Answer TypeState the law of constant composition or Definite proportion. Explain with suitable examples.
In an experiment 4.68 g iron oxide on reduction with hydrogen yields 3.86 g of iron. In another experiment 3.88 g of iron oxide gives 3.2 g of iron on reduction with hydrogen. Prove that the above data illustrates the law of constant proportions.
Weight of copper oxide obtained by treating 2.16 g of metallic copper with nitric acid and subsequent ignition was 2·7g. In another experiment, 1 · 15 g of copper oxide on reduction yielded 0·92g of copper. Show that the result illustrates the law of definite proportion.
