 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeClassify the following as pure substances or mixtures: air, milk, graphite, diamond, gasoline, tap water, distilled water, oxygen, one rupee coin, 22 carat gold, steel, iron, sodium chloride, iodized table salt.
Separate the following substances into elements and compounds: Graphite, Diamond, Distilled water, Oxygen, Iron, Sodium chloride, Blue vitriol.
How will you separate the following mixtures?
(i) Oil and water
(ii) Iron-filings and saw dust.
(iii) Glass powder and sugar.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeIf 6.3g of NaHCO3 are added to 15.0 g of CH3COOH solution, the residue is found to weigh 18.0g. What is the mass of CO2 released in the reaction?
The reaction is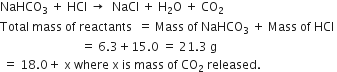
According to the law of conservation of mass:
Total mass of reactants = Total mass of products
21.3 = 18.0 + x
or x = 21.3 - 18.0 = 3.3 g
 Long Answer Type
Long Answer TypeState the law of constant composition or Definite proportion. Explain with suitable examples.
In an experiment 4.68 g iron oxide on reduction with hydrogen yields 3.86 g of iron. In another experiment 3.88 g of iron oxide gives 3.2 g of iron on reduction with hydrogen. Prove that the above data illustrates the law of constant proportions.
Weight of copper oxide obtained by treating 2.16 g of metallic copper with nitric acid and subsequent ignition was 2·7g. In another experiment, 1 · 15 g of copper oxide on reduction yielded 0·92g of copper. Show that the result illustrates the law of definite proportion.
