 Long Answer Type
Long Answer TypeHow Dalton's atomic theory was modified on the basis of modern reseraches? or Give various postulates of modern atomic theory.
Define Avogadro’s law. Taking a suitable example, prove that it is not in contradiction with Dalton’s atomic theory.
 Short Answer Type
Short Answer TypeDefine the following:
Average atomic mean.
Average atomic mass : The average atomic mass is defined as “the average relative mass of an atom of an element as compared to the mass of carbon atom (C-12) taken as 12 amu.’ For example, in naturally occurring neon isotopes and their relative abundance are as follows :
|
Isotope |
Fractional abundance |
|
20Ne |
0·9051 |
|
21Ne |
0·0027 |
|
22Ne |
0·0922 |
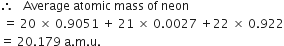
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeCalculate the atomic mass(average) of chlorine using the following data:
| %Natural Abundance | Molar mass | |
| 35Cl | 75·77 | 34·9689 |
| 37Cl | 24·23 | 36·9659 |
