 Short Answer Type
Short Answer TypeUse the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
Isotope Isotopic molar mass Abundance
36Ar 35·96755 g mol–1 0·337%
38Ar 37·96272 g mol–1 0·063%
40Ar 39·9624 g mol–1 99.600%
 Long Answer Type
Long Answer TypeCalculate the volume at STP occupied by:
(i) 16 g of oxygen
(ii) 2·5 moles of CO2
(iii) 1 × 1021 molecules of oxygen.
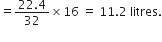
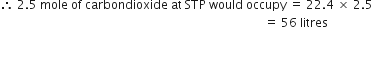
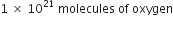

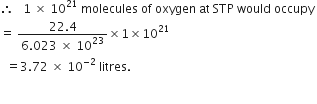
 Short Answer Type
Short Answer TypeIf 1021 molecules are removed from 200 mg of CO2, then how many moles of CO2 are left?
What is the name of the element whose mass of one atom is 4×10–23. Given Avogadro’s number as 6×1023.
 Long Answer Type
Long Answer TypeExplain the terms empirical and molecular formulae of a compound. How are these related to each other?
 Short Answer Type
Short Answer TypeCalculate the mass percentage of different elements present in sodium sulphate (Na2SO4).
