 Long Answer Type
Long Answer TypeA welding fuel gas contains carbon and hydrogen only. Burning a small space of it in oxygen gives 3.38 g carbon dioxide, 0·690g of water and no other products. A volume of 10·0L (measured at STP) of this welding gas is found to weigh 11·6g. Calculate: (i) empirical formula (ii) molar mass of the gas and (iii) molecular formula.
Butyric acid contains only C, H and O, 4.24mg sample of butyric acid is completely burnt. It gives 8.45mg of carbon dioxide and 3.46mg of water. What is the mass percentage of each element in butyric acid?
 Short Answer Type
Short Answer TypeIf the elemental composition of butyric acid is found to be 54.2%C, 9.2%H and 36.6%O, determine the empirical formula.
The molecular mass of butryic acid was determined by experiment to be 88 amu. what is the molecular formula?
 Long Answer Type
Long Answer TypeWhat are the implications of a chemical equation?
Or
What informations are conveyed by a chemical equation?
A chemical equation gives a lot of information viz.,
1. It tells the names of the reactants and products.
2. It tells the relative number of molecules of reactants and products.
3. It tells the relative number of moles of reactants and the products.
4. It tells the relative weights of reactants and the products.
5. It tells the relative volumes of reacting gases and those of products formed in case of gaseous reactants. For example, the chemical equation.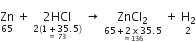
conveys the following information:
1. Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen.
2. One atom of zinc reacts with two molecules of hydrogen chloride to produce one molecule of zinc chloride and one molecule of hydrogen.
3. One mole of zinc reacts with two moles of hydrogen chloride to produce one mole of zinc chloride and one mole of hydrogen.
4. 65 gram of zinc reacts with 73 gram of hydrochloric acid to give 136 gram of zinc chloride and 2 gram of hydrogen.
5. 22·4 litres of hydrogen is produced at S.T.P. when one mole of zinc reacts with 2 moles of hydrogen chloride.
How can we make a chemical equation more informative?
Or
How can we remove the drawbacks or limitations of a chemical equation?
 Short Answer Type
Short Answer TypeWrite balanced chemical equation for the following reactions making them as informative as you can:
(i) Reaction between magnesium carbonate and dilute sulphuric acid to form an aqueous solution of magnesium sulphate, carbondioxide gas and water.
(ii) Reaction between methane gas (CH4) and oxygen gas to produce carbondioxide, water vapours and heat.
