 Long Answer Type
Long Answer TypeA welding fuel gas contains carbon and hydrogen only. Burning a small space of it in oxygen gives 3.38 g carbon dioxide, 0·690g of water and no other products. A volume of 10·0L (measured at STP) of this welding gas is found to weigh 11·6g. Calculate: (i) empirical formula (ii) molar mass of the gas and (iii) molecular formula.
Butyric acid contains only C, H and O, 4.24mg sample of butyric acid is completely burnt. It gives 8.45mg of carbon dioxide and 3.46mg of water. What is the mass percentage of each element in butyric acid?
 Short Answer Type
Short Answer TypeIf the elemental composition of butyric acid is found to be 54.2%C, 9.2%H and 36.6%O, determine the empirical formula.
The molecular mass of butryic acid was determined by experiment to be 88 amu. what is the molecular formula?
 Long Answer Type
Long Answer TypeWhat are the implications of a chemical equation?
Or
What informations are conveyed by a chemical equation?
How can we make a chemical equation more informative?
Or
How can we remove the drawbacks or limitations of a chemical equation?
The drawbacks of a chemical equation can be partially removed or we can make a chemical equation more informative as follows:
1. By specifying the physical states of the reactants and products in a chemical equation by putting the letter (s) for solid, (l) for liquid and (g) for gaseous state after the formula of the substance. The word (aq), which means aqueous solution, is used when the substance is in the form of solution, For example, reaction between sodium and water to form sodium hydroxide solution and hydrogen gas may be written as:
2. The concentrations of the reactants used in the form of solution can be shown by the word dil. for dilute and conc. for concentrated e.g.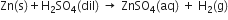
3. By using a down arrow  for the compound precipitated and an upward arrow
for the compound precipitated and an upward arrow  for a gas evolved. For example,
for a gas evolved. For example,
(i) Evolution of hydrogen gas.
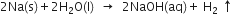
(ii) Precipitation of AgCl.

4. By mentioning the condition of reaction such as heat (), pressure, catalyst on the arrow head.
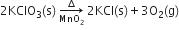
5. In case of exothermic reactions, the sign "+ heat" is written along with the products.

In case of enodthermic reactions, the sign "-heat" is written along with products. 
 Short Answer Type
Short Answer TypeWrite balanced chemical equation for the following reactions making them as informative as you can:
(i) Reaction between magnesium carbonate and dilute sulphuric acid to form an aqueous solution of magnesium sulphate, carbondioxide gas and water.
(ii) Reaction between methane gas (CH4) and oxygen gas to produce carbondioxide, water vapours and heat.
