 Long Answer Type
Long Answer TypeExplain the following terms with examples:
1. Skeleton equation
2. Balanced equation
3. Thermochemical equation.
 may or may not be equal.
may or may not be equal.
 For example, balanced equation representing the reaction between cupric oxide and ammonia is
For example, balanced equation representing the reaction between cupric oxide and ammonia is
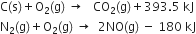
 Short Answer Type
Short Answer TypeWhat do you mean by 'balancing a chemical equation'? On what principle the balancing of a chemical equation is based?
 Long Answer Type
Long Answer Type