 Short Answer Type
Short Answer TypeHow many litres of oxygen at S.T.P. will be required to burn completely 2.2 g of propane?
Calculate the amount of KClO3 needed to supply sufficient oxygen for burning 112L CO gas at S.T.P.
How many moles of oxygen can be obtained by the decomposition of 1 mole of potassium chlorate in the following reaction:
How many moles of reactants are needed for obtaining 1 mole of oxygen?
Gastric juice contains 3g HCl per litre. If a person produces 2.5 L of gastric juice per day, how many antacid tablets each containing 400 mg of Al(OH)3 are needed to neutralise all the HCl produced in one day?
 Long Answer Type
Long Answer TypeCalculate the quantity of lime required to soften 60000 litres of well water containing 16.2 g of calcium carbonate per hundred litre.
 Short Answer Type
Short Answer TypeIf ten volumes of dihydrogen gas react with five volumes of dioxygen gas, how many volumes of water vapour could be produced?
 Long Answer Type
Long Answer TypeWhat volume of oxygen at STP is required to affect complete combustion of 200 cm3 of acetylene and what would be the volume of carbondioxide formed?
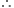 200 cm3 C2H2 will require O2 for complete combustion =
200 cm3 C2H2 will require O2 for complete combustion = 