 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA solution is prepared by dissolving 2g of a substance A to 18g of water. Calculate the mass percent of the solute.
Calculate the molarity of NaOH in the solution prepared by dissolving its 4g in enough water to form 250mL of the solution.
A solution is prepared by dissolving 5.3g of Na2CO3 in distilled water to give 500 mL of solution. Calculate the molarity of the solution.
A sample of NaNO3 weighing 0·38g is placed in a 50·0 mL volumetric flask. The flask is then filled with water to the mark on the neck. What is the molarity of the solution?
Molar mass of NaNO3 = 85 g mol-1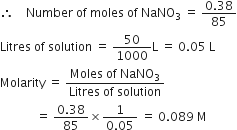
A sample of NaOH weighing 0.38g is dissolved in water and the solution is made to 50.0 mL in a volumetric flask. What is the molarity of the resulting solution?
