 Short Answer Type
Short Answer Type Multiple Choice Questions
Multiple Choice Questions20 mL solution of 0.1 M ferrous sulphate was completely oxidised using a suitable oxidising agent. What is the number of electrons exchanged?
1.204 x 1022
193
1930
1.204 x 1021
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat is the utility of mole concept?


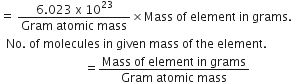
4. To calculate the number of molecules and the number of moles in a given mass of the substance.
No. of molecules in a given mass of a substance

5. To calculate the volume occupied at S.T.P. by a given mass of a gas.
Volume occupied at S.T.P. by given mass of a gas

6. To calculate the number of molecules present in a given volume of a gas at S.T.P.
No. of molecules contained in given volume of gas at S.T.P.

7. To calculate the number of atoms and molecules in the given number of moles of a substance.
No. of atoms in a given number of moles of element
= 6 · 023 × 1023 × No. of moles of element No. of molecules in a given number of moles of substance
= 6·023 × 1023 × No. of moles of substance
