 Short Answer Type
Short Answer Type In three moles of ethane (C2H6), calculate the following:
Number of moles of carbon atoms.
In three moles of ethane (C2H6), calculate the following:
Number of moles of hydrogen atoms.
In three moles of ethane (C2H6), calculate the following:
Number of molecules of ethane.
How many sulphur atoms are present in each of the following quantities
(i) 4.5 mole of S
(ii) 6 mole of S8 ?
 Long Answer Type
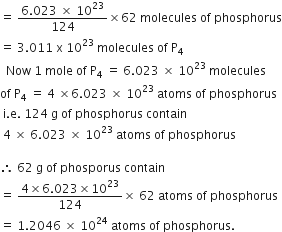
Long Answer TypeHow many atoms and molecules of phosphorus are present in 62 g of phosphorus (P4)?
Molecular formula of phosphorus = P4
Gram molecular mass of phosphorus (P4)
= 4 x 31 = 124 g
124 g of phosphorus contains 6.023 x 1023 molecules of phosphorus
![]() 62 g of phosphorus would contain
62 g of phosphorus would contain
Calculate the number of molecules present:
(i) in one drop of water having mass of 0.05 g
(ii) in 34.20 gram of cane sugar (C12H22O11).
 Short Answer Type
Short Answer Type