 Short Answer Type
Short Answer Type In three moles of ethane (C2H6), calculate the following:
Number of moles of carbon atoms.
In three moles of ethane (C2H6), calculate the following:
Number of moles of hydrogen atoms.
In three moles of ethane (C2H6), calculate the following:
Number of molecules of ethane.
How many sulphur atoms are present in each of the following quantities
(i) 4.5 mole of S
(ii) 6 mole of S8 ?
 Long Answer Type
Long Answer TypeCalculate the number of molecules present:
(i) in one drop of water having mass of 0.05 g
(ii) in 34.20 gram of cane sugar (C12H22O11).
(i) 1 mole of H2O = 18 g
= 6.023 x 1023 molecules
Mass of 1 drop of water = 0.05 g
Now 18 g of H2O contains 6.023 x 1023 molecules
![]() 0.05 g of
0.05 g of ![]() would contain
would contain
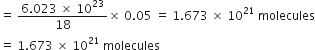
(ii) Molecular mass of ![]()
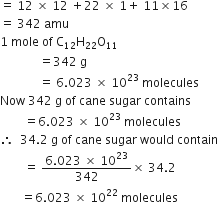
 Short Answer Type
Short Answer Type