 Short Answer Type
Short Answer TypeHow pressure of a gas is determined experimentally?
The pressure of a gas is measured with the help of an instrument called manometer. Two types of manometers are used:
(i) Open end manometer
(ii) Closed end manometer.
(i) Open end manometer. It consists of a V-shaped tube with one limb shorter than the other. It is partially filled with mercury. The mercury in the longer limb is subjected to the pressure of the gas in the vessel. Thus, the difference in the levels of mercury in the two limbs represents the difference between atmospheric pressure and gas pressure.
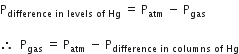
Thus, by knowing the atmospheric pressure, gas pressure can be determined.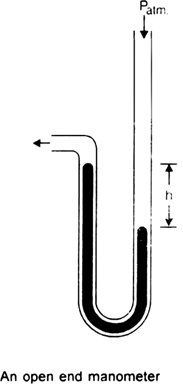
(ii) Closed end manometer: It consists of a V-shaped tube with one limb shorter than the other. It is partially filled with mercury. The shorter limb is connected to the vessel containing,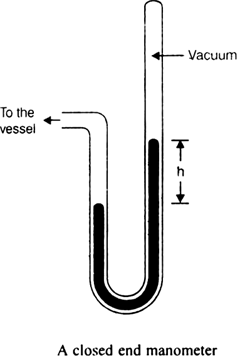
the gas whereas the longer limb is closed. In this manometer, the downward pressure exerted by the excess height of mercury column in the closed limb is balanced by the pressure of the gas in the vessel. Since there is a nearly complete vacuum in the closed longer limb, the difference in the levels of mercury in the two limbs directly gives the pressure of the gas.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeA manometer is connected to a gas containing bulb. The open arm reads 43.7 cm where the arm connected to the bulb reads 15.6 cm. If the barometric pressure is 743 nm mercury what is the pressure of the gas in a bar?
