 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are the units of pressure?
Pressure represents force per unit area. The pressure expressed in terms of height of mercury column can be converted into the units of force per unit area.
Let a mercury column h cm high and A cm2 in ara of cross-section exert a downward force equal to the weight of mercury column. Therefore, per unit area of the surface is P.

where m = mass of mercury in the column
g = acceleration due to gravity
But mass = density x volume
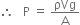
Where ρ and V represent density and volume of mercury respectively.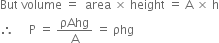
Standard atmospheric pressure. A standard pressure of one atmosphere (1 atm) is defined as the pressure exerted by a column of mercury 76 cm high at 273K (density of mercury is 13·595 g cm–3) and at standard gravity (i.e. 9·81 cm–2). That is,
1 atm = 76·0 cm of Hg = 760 mm of Hg = 760 torr.
The S.I. unit of pressure is pascal (Pa). It is defined as the pressure exerted when a force of 1 newton (1N) acts on a 1 m2 area. Pascal is related to atmosphere or bar as
1 bar =1 atm = 1 ·0133 × 105 Nm–2
= 1 ·0133 × 105 Pa = 101·33 k Pa
However, for approximate work,
1 bar 1 atm = 102 k Pa = 105 Pa
 Short Answer Type
Short Answer TypeA manometer is connected to a gas containing bulb. The open arm reads 43.7 cm where the arm connected to the bulb reads 15.6 cm. If the barometric pressure is 743 nm mercury what is the pressure of the gas in a bar?
